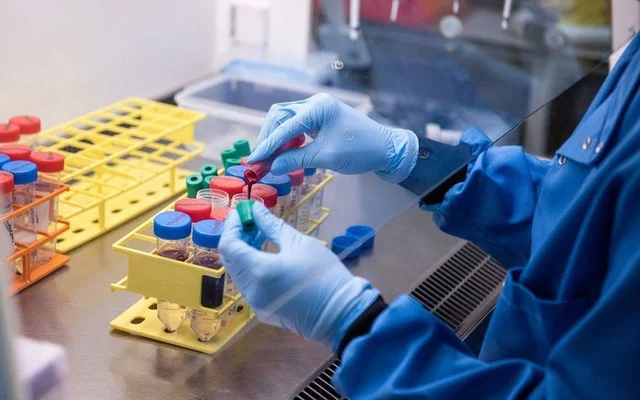
Antibodies generated by the vaccine being developed by the University of Queensland (UQ) and biotech firm CSL, one of four candidates contracted by the Australian government, were found to lead to some false positive HIV test results, the makers said.
While the vaccine had elicited a "robust" immune response to the novel SARS-COV-2 virus without serious adverse effects in a Phase 1 trial with 216 participants, re-engineering a fix could take another 12 months, they said.
CSL and the Australian government had together decided to stop Phase 2 and Phase 3 trials.
"While this is a tough decision to take, the urgent need for a vaccine has to be everyone's priority," said UQ professor Paul Young.
CSL, which had a contract to produce 51 million doses of the UQ vaccine, will instead produce an extra 20 million doses of the Oxford vaccine being developed with Britain's AstraZeneca , taking the total to 53 million